Preparing healthcare data for research and the next pandemic
One of the lessons of the COVID-19 pandemic is the need for clinical data fast to study disease patterns. Clinical data are essential not only in times of crisis but also during the ‘normal’ operation of the healthcare system for clinical trials, RWE, epidemiology, SDOH, the capacity of ER’s, etc...
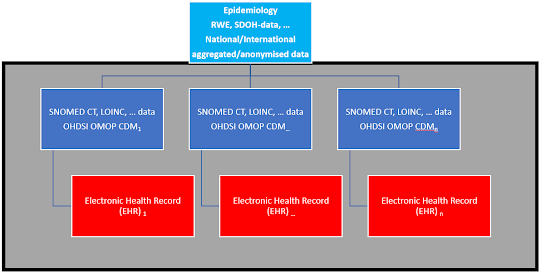
One way to achieve this is to use a federated system of OHDSI OMOP CDM databases, which mirrors the data of Electronic Health Records (EHR) in hospitals daily. By putting SNOMED CT, LOINC, and other standardized data in these databases in each hospital, the data can be analyzed with a standardized algorithm (R, Python, …), and the aggregated and anonymized data can be sent to national and international (EU) databases. This would avoid the chaos of standardizing data in a hurry when a health crisis arises (as was the case with COVID-19).
The healthcare community can regularly use the OHDSI OMOP CDM databases for clinical trials, Real World Evidence (RWE), etc., so they are not sitting idle and gathering dust. The OHDSI OMOP CDM databases create value for healthcare and society in everyday operations and during crises.
Of course, the data quality (complete, correct, unbiased) is essential, as, with all data-driven approaches, garbage in is garbage out (GIGO). Dealing with data in healthcare also means taking care of data safety, security, privacy, and confidentiality as crucial to dealing with healthcare data.